Tech Tip
At the ambient pH of most aquifers many dissolved metals (Ag, Ba, Cd, Cr, Cu, Fe, Li, Mn, Ni, Pb, Zn) are present as positively charged ions (cations). Transport of these dissolved cations is often controlled by sorption to negatively charged surfaces including sediment iron oxides (e.g., goethite, ferrihydrite, and hematite).
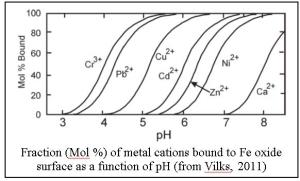
At low pH, iron oxides take on a positive charge and sorption of metal cations is low. However, as pH increases, the surface charge of iron oxides (and other minerals) becomes negative and sorption of metal cations increases. As a result, many metals sorb much more strongly at neutral to alkaline pH. The figure shows the expected distribution of several different metals to Fe oxide surface as a function of pH. For many cations, the extent of sorption increases rapidly over a narrow pH range, commonly referred to as a ‘sorption edge’.
Reference: Vilks, P., 2011. Sorption of Selected Radionuclides on Sedimentary Rocks in Saline Conditions – Literature Review, NWMO TR-2011-2012, Atomic Energy of Canada Limited.